The net heat added to the gas must equal work done by the gas, by conservation of energy. The work done by the gas during the process A to B is
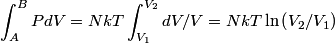 The work done by the gas during the process B to C is 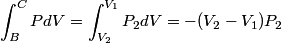 There is no volume change in going from C to A, so no work is done by the gas. So, the net heat added to the gas is  Therefore, answer (E) is correct. |